This web page was produced as an assignment for Genetics 677, an undergraduate course at UW-Madison.
Drug Interactions
An in vitro experiment done on human embryonic kidney (HEK) 293 cells showed that S-Nitrosylation of parkin inhibits parkin's ubiquitin E3 ligase activity and its protective function (1). The procedure involved the S-nitrosylation biotin switch assay, where HEK cells that were transfected with parkin were treated with S-nitrosoglutathione (GSNO). After parkin proteins were S-Nitrosylated by NO, they lost their ability to attenuate cell death initiated by synphilin-1.
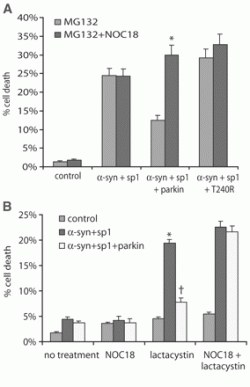
Figure 1: NO impairs the protective function of parkin (1). A) Alpha-synuclein and synphilin-1 induce a toxic effect on cells. Coexpression of parkin is able to attenuate the toxicity of alpha-synuclein and synphilin-1 in the presence of 10 µM MG132. The effect of parkin is lost in the presence of NOC18. B) Coexpression of parkin is able to attenuate the toxicity of alpha-synuclein and synphilin-1 induced by lactacystin. Once again, the effect of parkin is lost in the presence of NOC18
Figure 1: NO impairs the protective function of parkin (1). A) Alpha-synuclein and synphilin-1 induce a toxic effect on cells. Coexpression of parkin is able to attenuate the toxicity of alpha-synuclein and synphilin-1 in the presence of 10 µM MG132. The effect of parkin is lost in the presence of NOC18. B) Coexpression of parkin is able to attenuate the toxicity of alpha-synuclein and synphilin-1 induced by lactacystin. Once again, the effect of parkin is lost in the presence of NOC18
Analysis
The Chung et al. paper highlights the adverse effects that free radical stress has on neuron cells and their functions. Additionally, the findings establish a possible cause for parkin defects and the subsequent PD phenotype of lewy body formations. Further study into the effects that other small molecules have on parkin function should shed light onto the causitive agents of parkin downregulation and PD.
References
1. Chung, K., Thomas, B., Li, X., Pletnikova, O., Troncoso, J., Marsh, L., Dawson, V., Dawson, T. (2004). S-Nitrosylation of Parkin Regulates Ubiquitination and Comprimises Parkin's Protective Function.Science Vol. 304. pp. 1328 - 1331. DOI: 10.1126/science.1093891
